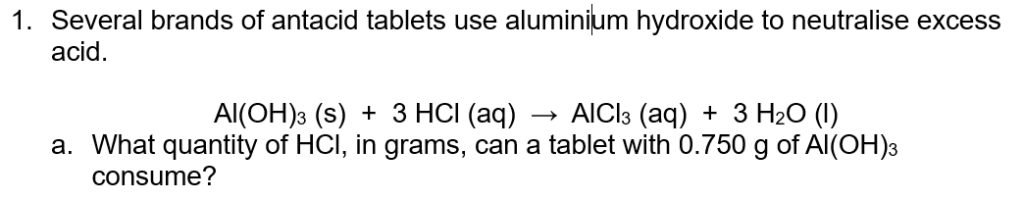
Q1b. What mass of water is produced?

Q2b. What mass of CO is required to reduce the iron (III) oxide to iron metal?
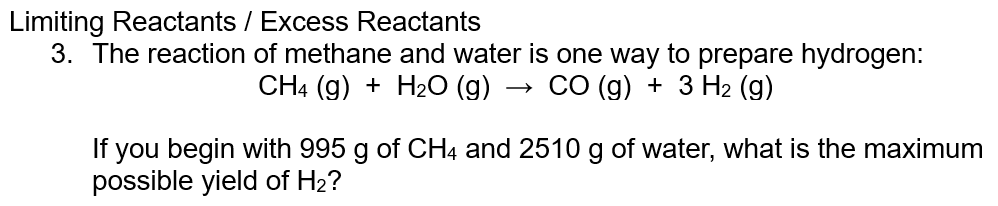

Q4b. What mass of S2Cl2 (in grams) can be produced?
In the video, changing the moles of S2Cl2 in grams is missed
Last step: moles of S2Cl2 x Mr so mass = 0.5 x 135 = 67.5 (Mr S2Cl2 = 67.5) Thanks for the feedback!!
4c. What mass of excess reactant remains when the limiting reactant is consumed?
In the video excess moles change to mass step is missed:
so; Excess mass = 0.5 x 71 = 35.5 g (Mr of Cl2 = 71)
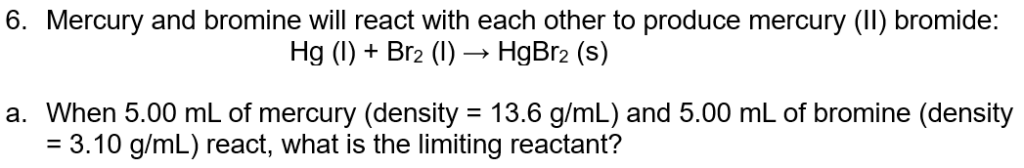
6b. How many grams of HgBr2 will be produced?
6c. How many ml of which reaction will be left over?


Very helpful